- DMTF 日本
- Home
- About DMTF
- Standards & Technology
- All DMTF Standard Publications
- Common Diagnostic Model (CDM)
- Desktop and mobile Architecture for System Hardware (DASH)
- Systems Management Architecture for Server Hardware (SMASH)
- Virtualization Management (VMAN)
- Common Information Model (CIM) Standards
- Configuration Management Database Federation (CMDBf)
- Platform Management Components Intercommunication (PMCI) Standards
- System Management BIOS (SMBIOS) Specification
- Storage Management Initiative (SMI)
- Web-Based Enterprise Management (WBEM)
- Web Services Management
- Management Profiles
- Technologies Diagram
- Works in Progress
- Technology Submission and Feedback Portal
- Historical Documents
- Cloud
- DMI Self-Certification
- News & Events
- Learning Center
- Conformance
- Join Us
CDM Conformance Program
Common Diagnostics Model (CDM) Conformance Program
- Increase interoperability between multiple clients
- Provides means to check CDM implementations to conformance with DSP1002 standard
- Increases market visibility to certified and conforming products in the market place
- Promotes further industry adoption
- Strengthens confidence in DMTF standards
The CDM Conformance Program (CDM CP) is designed to validate CDM implementations to a particular version of the CDM Implementation Requirements Specification and is managed and sponsored by the CDM Forum. CDM is used to evaluate the health of computer system components in multivendor environments. It specifies diagnostics instrumentation that can be utilized by vendors (OEMs and system builders) and platform management applicants to determine the health of a computer system components.
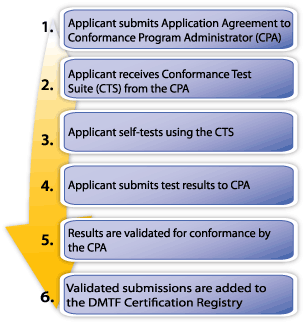
Companies interested in participating in the CDM CP self-test their implementation using the applicable CDM Conformance Test Suite and submit their digitally signed results to the CDM Conformance Program Administrator (an independent third party) for validation. The results will be validated by the Conformance Program Administrator. Certified results may be submitted for inclusion in the DMTF Certification Registry.
Click here to review the latest DMTF CDM Certified products.
The CDM Conformance Process
 |
"Products conforming to the CDM standard provide a consistent interface for cross-platform diagnostics enabling vendors to offer the best possible solutions for their customers. By providing a conformance program for vendors to test conformance to CDM Standards, DMTF continues enabling state-of-the-art interoperable management solutions for the industry." - Thomas Butler DMTF Vice President of Interoperability
|
CDM Conformance Test Suite
The CDM 1.0 Conformance Test Suite (CTS) software is provided by DMTF to industry leading vendors developing diagnostics within the DMTF DSP 1002 Profile Specification 1.0 using the CIM-XML protocol. Note: a WS-Management protocol version of the CTS will be available in the near future. Two versions of the CTS are available: Registered and Trial.
Results generated by the Registered version of the CTS qualify for validation by the DMTF CP Administrator. Successfully validated test results are eligible for inclusion in the DMTF CDM Forum Conformance Registry. The Registered version software of the CTS is free for DMTF members. There is a fee for non-members to use the tool.
The CTS Trial software version is offered by DMTF as a tool to assist diagnostic developers determine whether or not their diagnostics are within the framework of the DSP 1002 Profile Specification v1.0. The CTS Trial version software is not used to provide results to DMTF for certification or validation. An application to obtain a copy of the Trial version must be completed before the software can be received by the applicant.
If you would like more information on the CDM Conformance Program, or if your company is interested in testing its providers for CDM conformance, please contact the CDM Conformance Program Administrator via email.
CDM Certification Registry
Use the DMTF Certification Registry to search for CDM validated implementations.

